Fluidx Medical Technology has announced the results of the GPX-Clear embolic device in-vivo research which uses the base GPX technology and incorporates an intermediate-term radiopacity agent, as described by the company.
“Our initial in-vivo work with GPX-Clear looks very promising. It has the advantages of the baseline GPX product around ease of use, minimal preparation, and compatibility with standard microcatheters, with the added benefit of radiopacity that dissipates within the first 24 hours,” said Ryan O’Hara, an interventional oncologist at the University of Utah (Salt Lake City, Utah).
According to O’Hara, “There are many instances, particularly in oncology, in which clear visibility of the treated region post-embolisation is critical and can be obscured by radiopacity agents. This is an exciting future product in the GPX line-up and the first product of it’s kind.”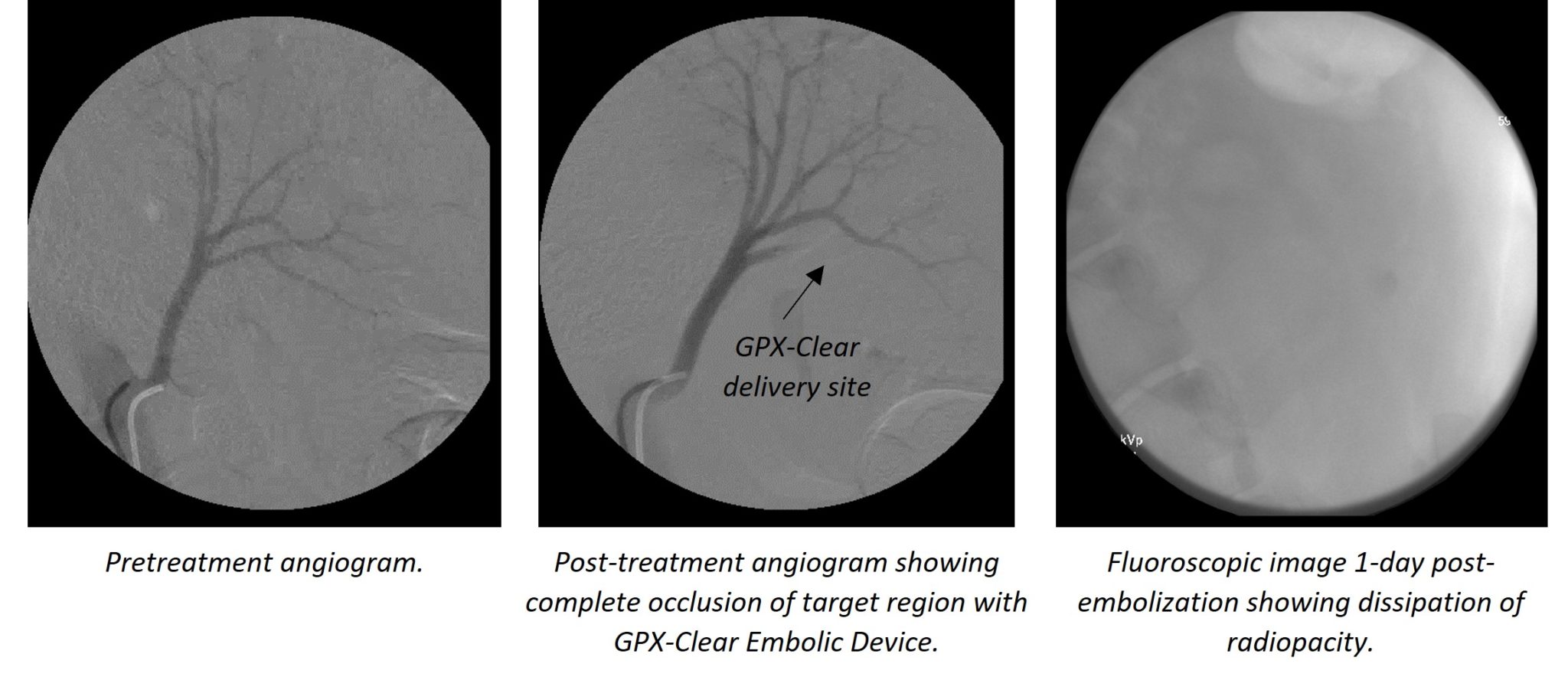
The GPX-Clear embolic device leverages the core GPX technology as well as incorporates a non-artifact-inducing radiopacity agent. The radiopacity agent is trapped within the polymer matrix, providing excellent visibility of the embolic device during and post-embolic delivery—the radiopacity agent then dissipates within 24 hours post-delivery, enabling unobstructed visibility of the treated area.
According to the company, the GPX embolic device is an innovative embolic designed for simple preparation and controlled delivery. The company describes that the device is packaged ready-to-use in a syringe, can be prepped tableside by the clinician in about 30 seconds, does not require dimethyl sulfoxide (DMSO) precipitation, and may be delivered through standard microcatheters. The company note, GPX is therefore designed to occlude blood vessels independent of a patient’s coagulation status.
The GPX embolic device is under development and does not have marketing clearance or approval in any market at this time.