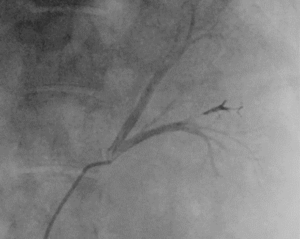
Fluidx Medical has released information about their new ULTRA embolic platform designed to make therapeutic embolization procedures safer, better, and faster, as the recent press release states.
Embolization is a minimally invasive catheter-based procedure that blocks blood flow to targeted blood vessels and organs. Over US$3.5 billion per year is spent on tiny embolic particles, metal coils, and liquid-to-solid devices to treat internal bleeding, aneurysms, fistula, uterine fibroids, tumours, prostates, malformations, and other conditions throughout the body.
“Embolic coils depend on the blood’s ability to clot and often require multiple coils to achieve complete occlusion,” said Ryan O’Hara, interventional radiologist and medical director at Comprehensive Integrated Care in Salt Lake City, Utah, USA. “Embolic particles are hard to visualise during delivery, increasing the risk of off-target embolization and leading to unintended harm of nearby tissues.”
The ULTRA platform is designed to address the needs of patients requiring moderately distal to proximal embolization. Unlike other embolic devices, ULTRA does not require refrigeration, use toxic dimethyl sulfoxide (DSMO), require special delivery catheters, need special mixers or preparation stations, or glue catheters into the body.
“We have seen a single delivery of ULTRA block a targeted vessel region quickly in an area that would usually require several coils,” noted Danny Smith, vice president of research and development at Fluidx Medical. “ULTRA is visible under X-ray giving clinicians greater control and visualisation of the embolic to ensure safe, accurate delivery.”
The success of the ULTRA embolic platform follows on the heels of another large milestone for Fluidx Medical, as their flagship product the GPX Embolic Device is currently under investigational device exemption (IDE) review with the US Food and Drug Administration (FDA). GPX, which completed a successful multicentre clinical trial as a multi-use embolic in 2023, focuses on embolization procedures where there is need for deep distal penetration.