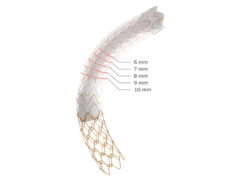
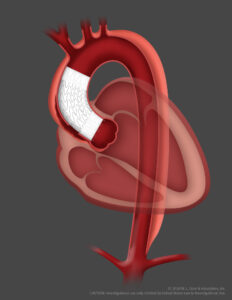
 Gore has announced the first patient implantation of the Gore ascending stent graft in the ARISE II trial, describing this as an exciting step in the development of treatments for pathologies involving the ascending aorta using endovascular repair rather than traditional open surgery.
Gore has announced the first patient implantation of the Gore ascending stent graft in the ARISE II trial, describing this as an exciting step in the development of treatments for pathologies involving the ascending aorta using endovascular repair rather than traditional open surgery.
On 1 December, national principal investigator Eric Roselli (Cleveland Clinic, Cleveland, USA) performed the case at Cleveland Clinic alongside study investigators Patrick Vargo and Frank Caputo. The patient was identified as a candidate for the study after presenting with a fusiform aneurysm of the ascending aorta and aortic arch.
The ARISE II study is the first multicentre pivotal study approved by the US Food and Drug Administration (FDA) investigating the use of a minimally invasive endovascular device to treat the ascending aorta. The study investigates the treatment of isolated lesions as well as chronic and residual type A dissections involving the ascending aorta. The Gore ascending stent graft is designed for investigational use in combination with the Gore Tag thoracic branch endoprosthesis.
“The treatment of the ascending aorta has long been a ‘final frontier’ in endovascular surgery. ARISE II is a significant step forward as we search for minimally invasive options that can be offered to higher risk patients,” said Roselli.
The ARISE II study will investigate how an endovascular stent graft, delivered via catheter, may be used to line the diseased portion of the ascending aorta as a potential alternative to open surgical repair. Endovascular technologies have been applied to other regions of the aorta to reduce the risk of complications and recovery times, but no endovascular device is currently approved for the ascending aorta.
“Our patient is recovering well. Having a minimally invasive alternative would be a significant advancement for patients not suitable for open surgery,” said Caputo.