BTG has announced the treatment of the first patient in Singapore with TheraSphere, a treatment for primary liver cancer and metastatic colorectal cancer, specifically engineered to deliver powerful doses of radiation to the tumour while minimising exposure to normal tissue in a procedure called radioembolization.
Stephen Chang, associate professor Liver, Tumour Group Lead and senior consultant, National University Cancer Institute, Singapore and National University Hospital, said: “Liver cancer is a deadly disease that impacts hundreds of people in Singapore each year and has a high mortality rate. This newly available therapy aims to destroy tumour cells, while maintaining the patient’s quality of life.”
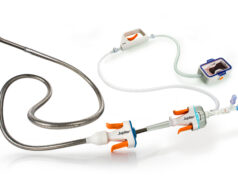
A press release from BTG notes that TheraSphere is a targeted therapy that consists of millions of glass microspheres containing radioactive yttrium-90. The microspheres are about 20-30 micrometers in diameter – about a third of the width of a human hair. They are delivered directly to liver tumours through the hepatic artery using a catheter. The glass microspheres flow directly into the liver tumour via its blood vessels and become permanently lodged there. Because the procedure delivers the treatment directly to the liver tumour, the radiation destroys the tumour cells with minimal impact to the surrounding healthy liver tissue. The radioactive microspheres continue to deliver radiation to the tumour over the course of several weeks after treatment. TheraSphere can also be used as a bridge to surgical removal of diseased tissue or transplantation in these patients, the release adds.
In Singapore TheraSphere is approved for both hepatocellular carcinoma, and metastatic liver cancer or cancer that has spread to the liver from another point of origin. Distributed in Singapore by Transmedic PTE, TheraSphere is also commercially available in Hong Kong. Over 18,000 patients worldwide have been treated with TheraSphere.
The National University Hospital in Singapore, and more than 20 hospitals across Asia, will also be participating in three international phase III clinical trials of TheraSphere.
- The EPOCH trial, evaluating TheraSphere in patients with metastatic colorectal carcinoma (mCRC) of the liver who have failed first-line chemotherapy.
- The STOP-HCC trial, evaluating TheraSphere in the treatment of patients with unresectable hepatocellular carcinoma.
- The YES-P trial, evaluating TheraSphere vs. the standard of care (sorafenib) for the treatment of advanced hepatocellular carcinoma with portal vein thrombosis.