The RADIANCE-II study of the Paradise Ultrasound Denervation System (ReCor Medical) for the treatment of hypertension has been approved by the US Food & Drug Administration (FDA).
Building upon the recent positive results of the RADIANCE-HTN SOLO study, RADIANCE-II will be a randomised, sham-controlled, blinded study in patients with moderate hypertension, powered to demonstrate the safety and efficacy of the Paradise System’s ability to lower blood pressure. ReCor expects to initiate enrolment in RADIANCE-II in October of 2018.
“We have had a very constructive dialogue with the FDA during the past nine months to craft what we believe is a strong study to develop a robust set of clinical data to support the future FDA review for PMA approval,” comments Leslie Coleman, vice president of Regulatory & Medical Affairs. “At the time of PMA submission we plan to have four independently-powered, blinded, sham-controlled, randomised studies of the Paradise System in patients with different stages of hypertension—our SOLO, TRIO, REQUIRE, and RADIANCE-II studies—approaching a total of nearly 500 patients, with outcomes as long as three years.”
“Our Steering Committee and medical advisors have been instrumental in the design of the Global RADIANCE Clinical Program—including the RADIANCE-II pivotal study—for the USA, Europe, Japan and Korea,” adds Helen Reeve-Stoffer, vice president of Clinical Affairs. “Given the recent positive SOLO results, and subsequently the numerous review articles in medical journals, we recognise the potential impact the Paradise System may have in the treatment of hypertension for millions of patients world-wide. Accordingly, ReCor is committed to conduct rigorous, randomised, controlled studies to demonstrate the safety and efficacy of the Paradise System to lower blood pressure, thus helping physicians to evaluate how, in whom, and when to use Paradise for the treatment of hypertension.”
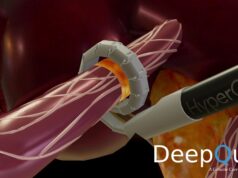
Paradise is an ultrasound-based system for endovascular denervation of the renal nerves Renal denervation is a potential therapeutic option for treatment of hypertension, one of the most prevalent medical conditions. The Paradise System bears a CE mark but is not approved for sale in the USA. ReCor Medical is conducting the RADIANCE-HTN clinical trial under an IDE from the US FDA in the USA and Europe, the REQUIRE trial in Japan and Korea with its partner Otsuka, and recently received approval from the FDA to conduct RADIANCE-II as a pivotal study of the Paradise System.