Delcath Systems has shared in a press release the publication of updated results from the phase 1b CHOPIN Trial, conducted at Leiden University Medical Center (Leiden, The Netherlands) on the use of the Delcath Chemosat hepatic delivery system with melphalan (Chemosat) in combination with the immune checkpoint inhibitors (ICI) ipilimumab and nivolumab to treat patients with metastatic uveal melanoma with liver metastases.
Delcath Systems has shared in a press release the publication of updated results from the phase 1b CHOPIN Trial, conducted at Leiden University Medical Center (Leiden, The Netherlands) on the use of the Delcath Chemosat hepatic delivery system with melphalan (Chemosat) in combination with the immune checkpoint inhibitors (ICI) ipilimumab and nivolumab to treat patients with metastatic uveal melanoma with liver metastases.
The publication is titled ‘Combining melphalan percutaneous hepatic perfusion with ipilimumab plus nivolumab in advanced uveal melanoma: First safety and efficacy data from the phase 1b part of the CHOPIN trial’ and was published in CardioVascular Interventional Radiology (CVIR).
Updated CHOPIN phase 1b trial results
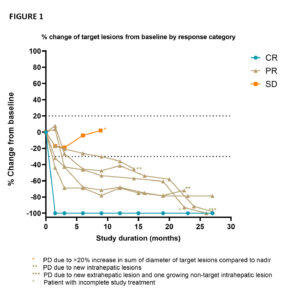
The goal of the CHOPIN trial is to study the safety and potential synergistic effects of systemic ICI therapy ipilimumab plus nivolumab (IPI+NIVO) when combined with Delcath’s proprietary liver-targeted percutaneous hepatic perfusion (PHP) treatment in metastatic uveal melanoma patients. The phase 1b portion of the trial enrolled seven patients, each of whom were treated with two courses of PHP (melphalan 3mg/kg, max. 220 mg per cycle) combined with four courses IPI+NIVO escalating the dosing from 1mg/kg each IPI+NIVO (cohort 1) to IPI 1mg/kg + NIVO 3mg/kg (cohort 2). The best overall response included one complete response, five partial responses and one stable disease accounting for an objective response rate of 85.7% and a disease control rate of 100% (figure 1). At the cut-off date of 15 November 2022, the median follow-up was 29.1 months (range 8.9–30.2), the median progression-free survival was 29.1 months (95% confidence interval [CI] 11.9–46.3) and the median duration of response was 27.1 months (range 7.4–28.5).
The ongoing randomised phase 2 part of the CHOPIN trial comparing M-PHP alone with M-PHP plus IPI+NIVO, which will include another 76 patients (38 per arm), is approximately 50% enrolled and will provide more insight to the efficacy.
The determination of a safe and effective dose was a primary goal of the Phase 1b portion of the CHOPIN trial. Grade 1/2 adverse events were seen in all patients and 71.4% experienced grade 3/4 toxicities. In this phase 1b dose-escalation study combining M-PHP with IPI+NIVO, the safe treatment dose was established at IPI 1mg/kg and NIVO 3mg/kg. The phase 2 part of the CHOPIN study will provide more information on both hepatic and systemic toxicity associated with the combination therapy.
“If similar results are observed in the larger, randomised, second phase of this trial, it would represent a meaningful improvement over current treatment options for this patient population,” said Johnny John, the company’s senior vice president, Medical and Clinical Affairs. “In addition, further investigation of this combination protocol may be warranted in patients with liver dominant disease in other tumour types currently treated with ICI agents.”
Rationale for combination therapy
The rationale for combining ICI therapy with M-PHP in metastatic uveal melanoma is based on both uveal melanoma’s specific characteristics and the unique immunomodulatory role of the liver. Uveal melanoma is characterised by a different set of driver mutations and lower mutational load compared to cutaneous melanoma, leading to limited neoantigen presentation and lower efficacy of ICI. By combining M-PHP with IPI+NIVO, the authors noted that they aim to increase the efficacy of ICI by turning a ‘cold tumour’ into a ‘hot tumour’. In addition, while PHP can provide long-lasting disease control in the liver, it does not control extrahepatic disease. Conversely, IPI+NIVO treatment shows a trend towards control of extrahepatic lesions, but hepatic disease progression regularly occurs. With combined treatment, the authors aim to control hepatic disease, as well as prevent extrahepatic disease in follow-up. In addition, it is well documented that the liver has a unique immune-modulating role and liver metastases diminish ICI efficacy systemically regardless of the type of primary tumour and in animal models, it has been shown that this effect can be overcome by localised hepatic therapy Current available evidence from studies on isolated limb perfusion (ILP) and isolated hepatic perfusion (IHP), which is the surgical counterpart of M-PHP, show that ILP and IHP can lead to T-cell activation following the procedures. The authors hypothesise that this is also the case for M-PHP leading to an improved activation of the immune system together with ICI.