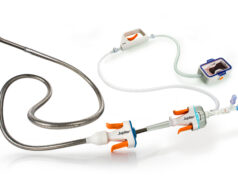
Cook Medical has received approval from Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) to market the Zilver PTX drug-eluting peripheral stent in Japan. The device, indicated for treating peripheral artery disease in the superficial femoral artery, is the first stent available in Japan approved for use in the superficial femoral artery, according to a company release. Its approval also makes Zilver PTX the only drug-eluting peripheral stent available in Japan.
“The Zilver PTX peripheral stent represents progress in treating peripheral artery disease, and the Japanese government should be proud of the commitment it has shown to patients with this timely approval,” said Rob Lyles, vice president and global leader, Cook Medical’s Peripheral Intervention division. “Drug elution has come to the periphery for a reason. Clinical trials show that Zilver PTX has better long-term patency outcomes than bare-metal stents.”
Mitsuo Asami, vice president, Cook Japan and leader of Cook Japan’s Peripheral Intervention division added: “We are proud that this approval will bring Zilver PTX to Japanese physicians and patients. We intend to continue to support all medical practitioners by providing safe and minimally invasive treatment options for peripheral artery disease.”
In a cooperative, multinational regulatory effort, a clinical trial for this product was carried out by Cook in Japan, the United States and Germany, with data from the trials being used to support regulatory submissions for Zilver PTX in Japan, the USA and Europe.
The Zilver PTX drug-eluting stent is under US Food and Drug Administration (FDA) review and is not available for sale in the United States. It received CE mark approval in August 2009. The device is now available in more than 45 countries.