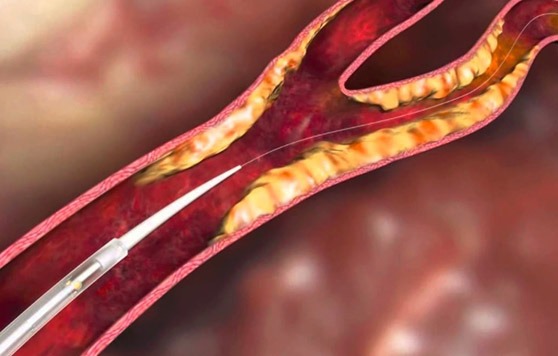
 InspireMD has announced positive interim results from the SIBERIA trial, an investigator-initiated study of the CGuard embolic prevention system (EPS). The data were presented at the Leipzig Interventional Course (LINC; 22–25 January, Leipzig, Germany).
InspireMD has announced positive interim results from the SIBERIA trial, an investigator-initiated study of the CGuard embolic prevention system (EPS). The data were presented at the Leipzig Interventional Course (LINC; 22–25 January, Leipzig, Germany).
Andrey Karpenko and Pavel Ignatenko from the Siberian Federal Biomedical Research Center, Russia, presented the interim data from their first 50 patients in an independent, randomised clinical trial designed to ultimately include 100 consecutive patients with symptomatic and asymptomatic carotid artery disease. The patients are randomly assigned (1:1) into two treatment groups. Fifty patients will receive the CGuard EPS MicroNet mesh covered stent and the other 50 patients will receive a conventional carotid stent. The primary endpoints are the incidence and volume of new lesions within the brain after carotid stenting using diffusion weighted magnetic resonance imaging (DW-MRI) peri-procedurally and at 30 days.
At the protocol-mandated interim analysis, after recruiting the first 50 patients in the study, the results at one month show that, despite having patients with higher risk factors in the CGuard group compared to the conventional carotid stent arm, the CGuard-treated patients had a significantly lower incidence of multiple lesions in the brain (16% vs. 44%), and a lower incidence of large cerebral lesions (24% vs. 40%). Finally, major adverse clinical events after 30 days occurred in the conventional carotid stent arm but not in the CGuard EPS-treated patients (12% vs. 0%).
Karpenko commented, “We are excited that despite the higher-risk patient profile in the CGuard group, the interim results show a significant reduction of cerebral embolisation while patient outcomes suggest a clinically-relevant benefit of the CGuard EPS. This mandated interim analysis gives us confidence to continue enrolling patients in this trial and extending the data-set in this potentially important advancement in the stroke prevention field.”








