Boston Scientific has signed an agreement to acquire Veniti, a privately-held company in Fremont, USA which developed and commercialised the Vici venous stent system for treating venous obstructive disease. Boston Scientific has been an investor in Veniti since 2016 and currently owns 25% of the company. The transaction price for the remaining stake consists of US$108 million up-front cash, as well as up to US$52 million in payments contingent upon US Food and Drug Administration (FDA) approval of the Vici stent system.
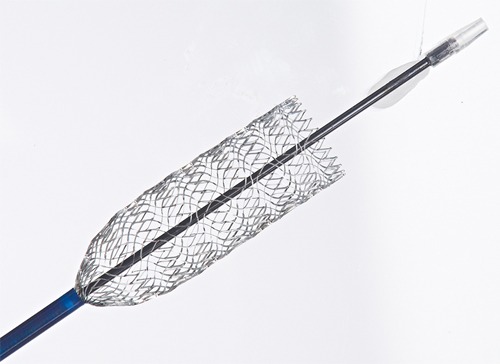
The self-expanding, nitinol Vici stent system was developed specifically for use in the venous anatomy, which presents different challenges than placing stents in the arterial vascular system. The Vici stent is designed to withstand compression and maintain patency and flexibility over the course of a patient’s life expectancy.
“This stent system was designed with the distinctive demands of the venous system in mind, and built to provide physicians with a high-quality lumen across a variety of venous anatomies and disease states,” said Jeff Elkins, president and CEO of Veniti. “We are excited to see this stent technology become even more accessible to physicians and the patients they treat under the leadership of Boston Scientific.”
The Vici stent system received CE Mark in 2013 and Veniti submitted a pre-market approval (PMA) application to the FDA in June, leveraging results from the recently completed VIRTUS pivotal study. Currently in the USA, there are no stent technologies specifically indicated for use in the peripheral venous system.
“With the unique benefits of this differentiated technology and the strong experience of Boston Scientific in the overall venous market, we believe the Vici stent will become an important choice for physicians who choose stents to treat patients suffering from venous disease,” said Jeff Mirviss, senior vice president and president, Peripheral Interventions, Boston Scientific. “Along with our leading AngioJet thrombectomy platform and venous product pipeline, we look forward to meeting the needs of physicians treating both chronic and acute venous disease.”
The acquisition of Veniti is expected to be immaterial to Boston Scientific adjusted earnings per share (EPS) in 2018 and 2019, and accretive thereafter. On a GAAP basis for 2019 and subsequent years, the transaction is expected to be less accretive, or more dilutive as the case may be, due to amortisation expense and acquisition-related net charges. For 2018 on a GAAP basis, the transaction is expected to be accretive due to a one-time gain on prior investment. The completion of this transaction is imminent, subject to customary closing conditions.
In the USA, the VICI Stent System is an investigational device and is not available for sale.