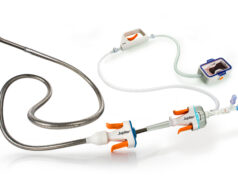
ArtVentive Medical Group has announced that it has received the CE mark for the ArtVentive EOS peripheral vascular enoluminal occlusion system.
“This is a very significant achievement in the company’s history as it moves toward commercialisation and its planned launch into the European markets,” said Leon Rudakov, president and CTO.
“The CE mark also paves the way to facilitate approvals for the ArtVentive EOS device internationally in meeting with the company’s global mandate,” stated Jim Graham, CEO and chairman.