 The Tack Optimised Balloon Angioplasty II below-the-knee (TOBA II BTK) clinical trial has successfully completed enrolment, ahead of schedule. This study, sponsored by Intact Vascular, is the first pivotal trial investigating a permanent vascular implant in arteries below the knee.
The Tack Optimised Balloon Angioplasty II below-the-knee (TOBA II BTK) clinical trial has successfully completed enrolment, ahead of schedule. This study, sponsored by Intact Vascular, is the first pivotal trial investigating a permanent vascular implant in arteries below the knee.
The study will examine the safety and efficacy of the Tack endovascular system (Intact Vascular) when used to repair dissections in the arteries below the knee following percutaneous transluminal angioplasty as a treatment for critical limb ischaemia (CLI).
“Patients with CLI experience painful symptoms and are at increased risk of amputation. Unfortunately, therapeutic options are very limited, and no scaffolding solutions are currently FDA-approved for BTK interventions,” commented George Adams, director of Cardiovascular and Peripheral Vascular Research, UNC Rex Hospital, Raleigh, USA, and co-principal investigator for the TOBA II BTK study. “The potential to have a treatment option that maintains vessel integrity and improves blood flow will have a significant clinical impact for treating patients with below-the-knee disease.”
With enrolment completed at 41 US and European sites, the TOBA II BTK trial is a single arm, prospective study to investigate the safety and efficacy of the Tack endovascular system for the repair of post-angioplasty dissections in the mid/distal popliteal, tibial and peroneal arteries. All 233 patients enrolled suffered from CLI, underwent standard balloon angioplasty, and consequently experienced at least one dissection requiring repair.
“Dissections are an expected consequence from balloon angioplasty, yet can have significant implications for patients,” states Patrick Geraghty, professor of Surgery and Radiology at the Washington University School of Medicine in St Louis, USA and co-principal investigator for the TOBA II BTK trial. “Early results with the Tack implant are promising, and I look forward to integrating this technology into my below-the-knee treatment algorithm.”
“Reaching full enrolment for our TOBA II BTK study is an important milestone for below-the-knee interventions,” comments Peter Schneider, a vascular surgeon, and co-founder and Chief Medical Officer of Intact Vascular. “As the first dissection repair device purpose-built for use in these small vessels, we are excited to provide a solution for patients suffering the painful and debilitating effects of CLI and who currently do not have adequate treatment options available. The Tack implant is a novel adjunct therapy that should diminish the chance that these patients will require an amputation.”
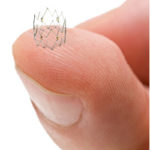
The Tack endovascular system is designed to be used in conjunction with peripheral balloon angioplasty in the treatment of peripheral arterial disease. The system is pre-loaded with six self-expanding nitinol devices for above-the-knee interventions, or four for below-the-knee, and can be deployed to treat multiple dissections using a single catheter.
Intact Vascular is sponsoring three clinical trials to evaluate its Tack endovascular system: TOBA II, TOBA II BTK and TOBA III. TOBA II is investigating the combination of the Tack implant with plain angioplasty balloons and the BD Lutonix DCB in arteries above the knee, and successfully achieved both primary and secondary endpoints, as reported at VIVA (5–8 November, Las Vegas). TOBA II BTK is investigating the combination of the Tack implant with plain balloon angioplasty in the arteries below the knee. TOBA III has completed enrolment in Europe and is investigating the combination of the Tack implant with the Medtronic IN.PACT Admiral DCB, inclusive of long lesions.
The original TOBA BTK study in 2016 reported positive six-month results, with a primary patency at that time-point of 87.1%.









