 Guerbet has announced the registration of Vectorio, a kit for imaging hepatocellular carcinoma (HCC), in Canada.
Guerbet has announced the registration of Vectorio, a kit for imaging hepatocellular carcinoma (HCC), in Canada.
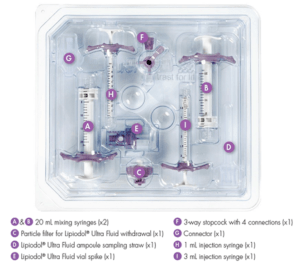
The kit consists of a set of Lipiodol-resistant medical devices that includes patented syringes, stopcock and sampling devices.
Vectorio is dedicated to Lipiodol Ultra Fluid Injection for imaging of liver tumours in adults with known HCC during interventional oncology procedures.
HCC is the most common primary liver cancer and is the fourth biggest cause of death due to cancer worldwide.
This medical device:
- Is 24 hours Lipiodol Ultra Fluid resistant
- Contains Patented three-way stopcock with four connections offering possibility of “On-table Refill and optimised injection control” (interventional radiologists have the possibility of refill without disconnection from the micro-catheter)
- Includes all devices in one set
- Is user-friendly and allows ergonomic and quick device set-up.
Designed and manufactured in France, the product was launched commercially in September 2017 in Europe. Today, Vectorio for HCC imaging is registered in Canada and Germany. Vectorio is also registered in 25 countries where conventional transarterial chemoembolization (cTACE) procedures are approved for Lipiodol Ultra Fluid.













