Personalised treatment selection and planning based on controlled dosimetry will lead to improvements in treatment accuracy. This is the conclusion presented by Marnix Lam (University Medical Center Utrecht, Utrecht, The Netherlands) at ECIO Virtual (27 January, online), a series of afternoon webinars that the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) is hosting in lieu of the 2020 European Conference on Interventional Oncology (ECIO). Lam spoke on the differences between Yttrium-90 (Y-90) and Holmium-166 (Ho-166) radioembolization, and told delegates that it was “very important” to ensure interventionalists conduct a “good” scout procedure with control over selection and planning.
Currently, there are three microspheres available for radioembolization treatment in Europe: TheraSphere (Boston Scientific), SIR-Spheres (Sirtex), and Quiremspheres (Terumo). Lam began his talk by highlighting several differences between these microspheres. “Firstly,” he said, “the isotope. This is Y-90 for TheraSphere and SIR-Spheres, and Ho-166 for Quiremspheres.”
Importantly, he said, the different isotopes have different characteristics. Relative embolic effect is lowest for TheraSphere, middling for Quiremspheres, and highest of the three for SIR-Spheres. This is because the number of particles that are injected differs for each microsphere brand: five million for TheraSphere, 50 million for SIR-Spheres, and 20 million for Quiremspheres.
Lam explained: “Typically for Theraspheres, there is a low embolic effect, because we only administer around five million particles. With SIR-Spheres, there is a higher embolic effect, because we administer up to 50 million particles, ten times the number of particles as with TheraSphere. With Quiremspheres, we are somewhere in between, with a medium embolic effect, as we administer around 20 million particles. This means that the specific activity of TheraSphere is much higher: so, the activity per sphere is much higher for Therasphere than it is for SIR-Spheres, and Quiremspheres is again somewhere in between.”
He cited the specific activity for each microsphere as: 1,250–2,500Bq/microsphere for TheraSphere, 50Bq/microsphere for SIR-Spheres, and 200–400Bq/microspheres for Quiremspheres.
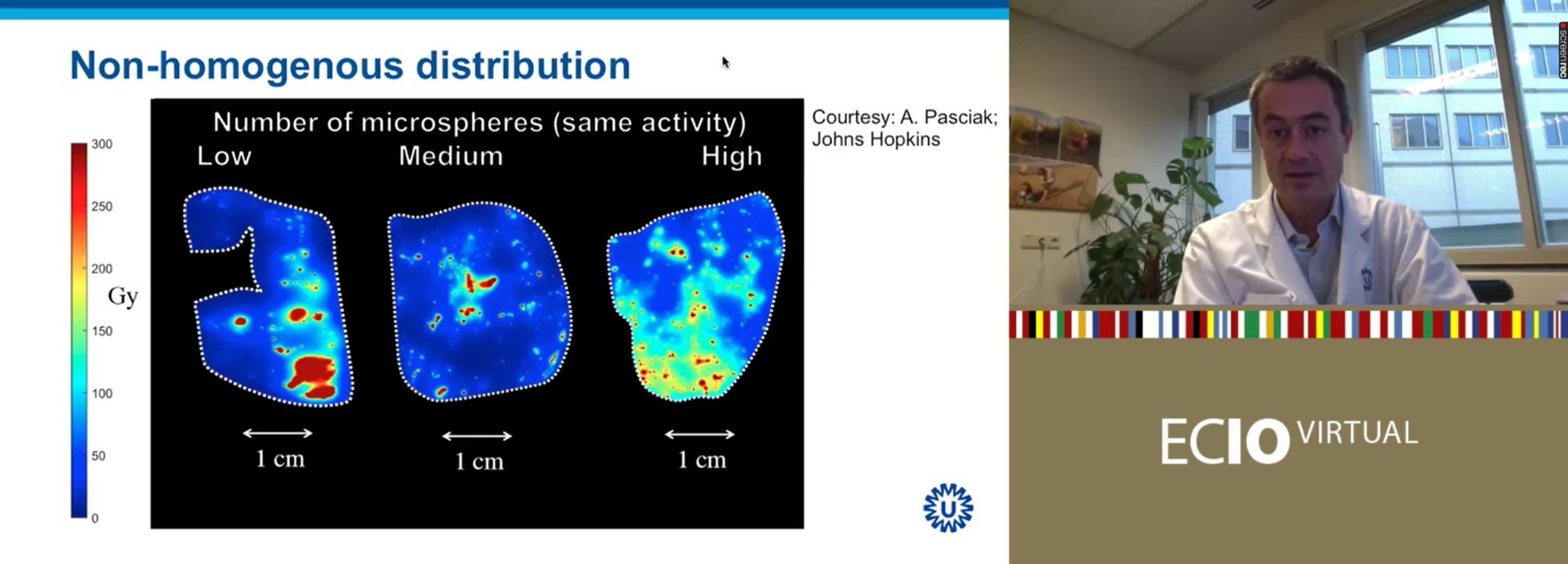
“What does this mean?” Lam asked rhetorically. “This is really important to understand”. Pre-clinical work by Alexander Pasciak (Johns Hopkins University, Baltimore, USA) et al in pig livers investigated the use of different numbers of microspheres over the same overall radiation activity (i.e., the same Gy in total; so, when few microspheres were used, they each have a high specific activity, and when large numbers of microspheres were used, they each had a low specific activity). When relatively few microspheres were injected, there was a very heterogeneous distribution pattern. In contrast, when a large number of microspheres were injected into the liver, each with a low specific activity, the distribution pattern was much more homogenous across the organ.
Liver tolerance of the same radiation activity (as measured in Gy), the same absorbed dose, is therefore greater when fewer particles are injected, each with a higher specific activity. The more homogenous distribution of radiation in the high-number-of-microspheres-injected model means that there is a much more limited area of unaffected liver. As Lam explained at ECIO Virtual, in the low-number-of-microspheres group, certain areas of the liver are “super dead”—have a very concentrated radiation dose—and a large swathe of the liver is unscathed.
“This is exactly the difference between TheraSphere [low particle count] and SIR-Spheres [high particle count],” he said. QuiremSpheres are in between.
Expanding on what this means in terms of tolerability, Lam informed listeners that for glass microspheres, a 100Gy radiation dose to the liver results in a 50% chance of toxicity. The same toxicity risk is attained at a 50Gy radiation dose when using resin microspheres, “so half the absorbed dose to the liver, same chance of toxicity, because the distribution is more homogeneous”. The dose thresholds used for treatment planning therefore vary depending on what type of microsphere is used.
Lam commented that this “is a very important message”, and advised the audience “to be very careful when comparing doses between products”.
Interventionalists utilise different imaging modalities for different microspheres
Turning to imaging, Lam explained how, due to differing microsphere characteristics, the imaging modality used for each also varies. Expanding on the clinical implications of this, Lam explained: “The idea of using Ho-166 instead of Y-90 is that Ho-166 has a therapeutic effect very similar to Y-90, but on top of that the element Holmium, like Gadolinium, is chemically a lanthanide, so it is paramagnetic and may be visualised and quantified using MRI [magnetic resonance imaging]. Holmium also emits gamma radiation, that may be used for quantitative SPECT [single photon emission computed tomography] imaging.”
While Y-90 can be imaged using SPECT or positron emission tomography (PET), Lam told ECIO Virtual attendees that the former modality is limited by resolution, and the latter by sensitivity. However, Ho-166 can be imaged at low quantities by MRI or SPECT, meaning that with Quiremspheres, the same particles can be used for the scout dose as for the treatment dose, unlike with Theraspheres and SIR-Spheres. Lam went on to explain the clinical significance of this characteristic of Ho-166.
Typically, ahead of a radioembolization procedure, he explained, the interventionalist will conduct a work-up or scout procedure using Tc-99m MAA (macroaggregated albumin).
The 2017 SARAH trial, an open-label, randomised controlled phase 3 trial investigating the efficacy and safety of selective internal radiotherapy (SIRT) with Y-90 resin microspheres compared with sorafenib in hepatocellular carcinoma (HCC) patients, found no significant difference in survival between the two treatments. In a more recent post-hoc analysis of the SARAH trial, published in Radiology in 2000, Ann-Laure Hermann (Université Paris-Descartes, Sorbonne Paris Cité, Faculté de Médecine, Paris, France) et al showed that when the tumour-absorbed dose is at least 100Gy, there is a clear difference in survival between resin microspheres and SIRT. “I think there is quite a spectacular result when you compare groups over 100Gy tumour-absorbed dose or below 100Gy tumour-absorbed dose,” Lam noted.
“However,” he continued, the same study also demonstrated that the correlation between the Tc-MAA distribution and the post-treatment Y-90 distribution was “good in 53% of patients only”. In 20% of patients, there was a medium correlation, and in “as much as 27% of patients”, a correlation between Tc-MAA and Y-90 distribution was poor.
“This means if we use Tc-MAA for treatment planning, in only 53% of patients the predicted value of Tc-MAA versus post-treatment Y-90 is sufficient for treatment planning. So, this is quite poor,” Lam said. “What we learn from this study is that of course, number one, we have to select patients who will benefit: patients where we can reach a 100Gy absorbed dose, and of course we should plan our treatment accordingly, to make sure we reach that tumour-absorbed dose.
“But what we also learn from this study is that we gain control by having an improved scout procedure, where the work-up is predictive of the final outcome. I believe this is where Holmium steps in, because instead of Tc-MAA, we use the exact same particles for work-up as we use for the treatment procedure. What we gain is control—over the lung-shunt calculation, and also over the intra-hepatic distribution.”
Tomorrow’s treatment planning method: Individualised patient care
Looking to the future, Lam enthused about personalised treatment planning. “Today, we use single compartment modelling at best,” he said, “aiming for an average absorbed dose within a certain perfused volume, but we really want to go towards multi-compartment modelling, differentiating between a tumour-absorbed dose and a normal-liver-absorbed dose in each individual patient. personalised
“We can use the scout dose procedure, the imaging that we gain, by selecting our patients based on a safe normal-liver-absorbed dose and a sufficient tumour-absorbed dose. If we are not able to reach that, we should seek an alternative treatment; so, selection comes first, and, secondly, we should plan our treatment accordingly. This is very important.” personalised personalised personalised personalised personalised personalised personalised personalised













