Data from the EXRACT-PE trial, published online first in the Journal of the American College of Cardiology (JACC): Cardiovascular Interventions, found that the Indigo Aspiration system (Penumbra) met its predefined safety and efficacy endpoints for the treatment of pulmonary embolism (PE). In this multicentre, prospective study, the Indigo aspiration system was associated with a significant reduction in the right ventricular-to-left ventricular (RV/LV) ratio and a low major adverse event rate in submassive PE patients. Intraprocedural thrombolytic drugs were avoided in 98.3% of patients.
EXTRACT-PE stands for Evaluating the Safety and Efficacy of the Indigo aspiration system in Acute Pulmonary Embolism. It is a single-arm investigational device exemption (IDE) trial that enrolled 119 patients at 22 US sites, and is sponsored by Penumbra. Patients with signs and symptoms of acute PE for ≤14 days, computed tomography angiography (CTA) evidence of PE, systolic blood pressure ≥90 mm/Hg with evidence of dilated RV (RV/LV ratio >0.9), and who were 18 years of age or older were eligible for enrollment.
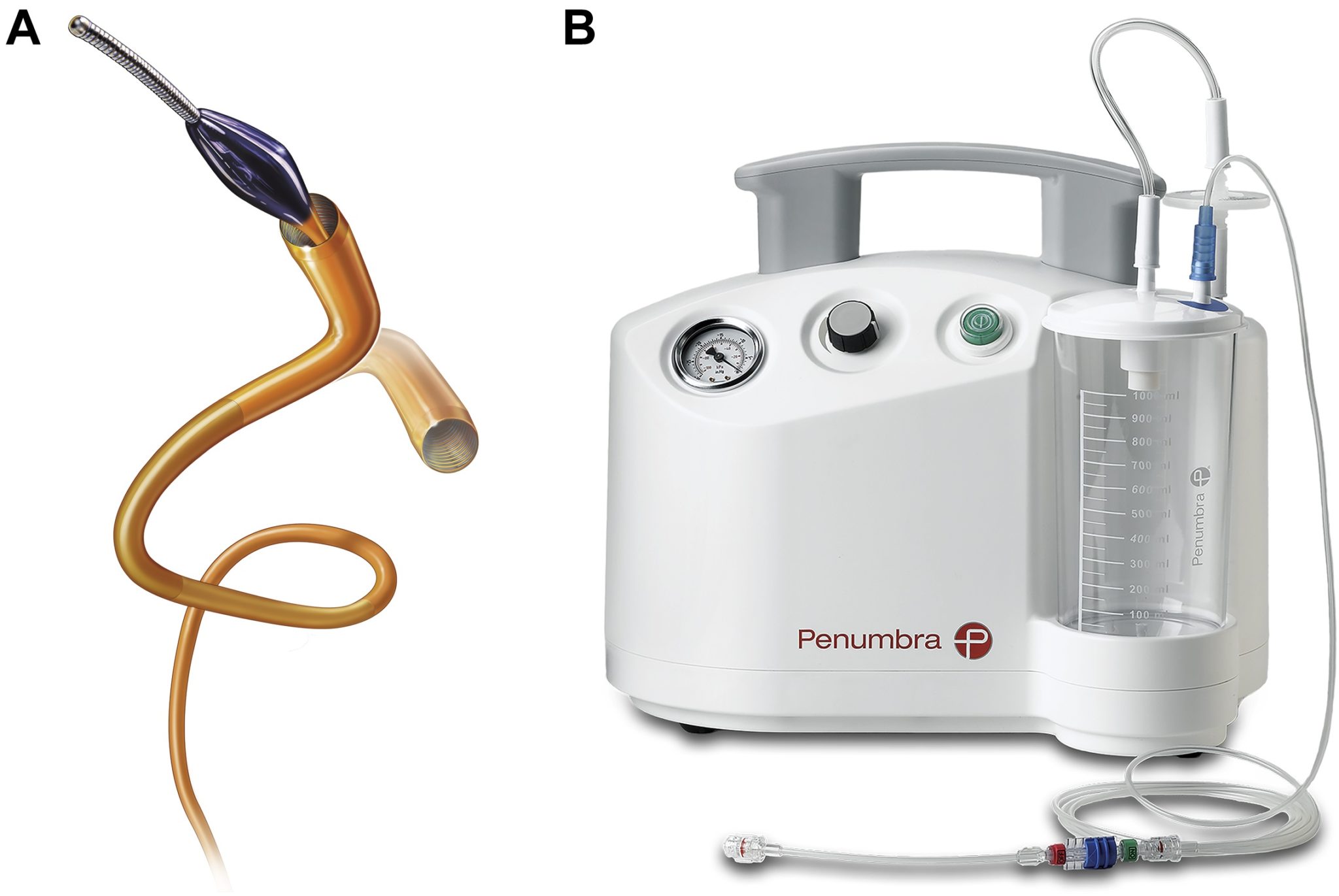
Patients were treated for acute PE with the Indigo aspiration system at each investigational sites. According to Penumbra, the Indigo system is designed to remove clots from arteries and veins in the peripheral vasculature. It was introduced in 2014, and received clearance for the PE indication in January 2020.
The primary efficacy endpoint analysis was the difference between the baseline and the 48-hour RV/LV diameter ratio; it was met if the lower bound of the confidence interval (CI) was >0.2.
The primary safety endpoint was the rate of major adverse events at the 48-hour mark, a composite of device-related death, major bleeding, and device-related serious AEs (SAEs). The primary safety endpoint was met if the upper bound of the CI was <40%. Device-related SAEs were a composite of clinical deterioration, pulmonary vascular injury, and cardiac injury. Secondary safety endpoints at 48 hours included rates of device-related death, major bleeding, clinical deterioration, pulmonary vascular injury, and cardiac injury. Additional secondary safety endpoints were 30-day rates of any-cause mortality, device-related SAEs, and symptomatic recurrence of PE.
Detailing their results, lead author Akhilesh K Sista (NYU Grossman School of Medicine, New York, USA) and colleagues write: “In this prospective study of the Indigo Aspiration device for submassive PE, we found a statistically significant reduction in RV/LV ratio (0.43; 95% CI: 0.38–0.47; p<0.0001), an average reduction in systolic pulmonary artery pressure of 7.9%, and a low major adverse event rate (1.7%) following use of the device. This was achieved with 98.3% of patients receiving no procedural tissue type plasminogen activator (tPA). The rate of major bleeding was 1.7%, and there were no haemorrhagic strokes. Of note, 89.6% (n=103 of 115) of patients had a reduction in RV/LV ratio without an adverse event related to the device, major bleeding, PE-related death, or clinical deterioration.”
This led them to conclude that the Indigo Aspiration system “may be considered by endovascular physicians for use in intermediate risk PE”, and to express the opinion that future prospective studies and randomised trials should focus on which patients (including high-risk PE patients and those with thrombolytic drug contraindications) derive the greatest benefit from aspiration thrombectomy.
Sista first presented these results at the 2019 Vascular Interventional Advances conference (VIVA; 4–7 November, Las Vegas, USA).