
This advertorial is sponsored by PharmaCept.
Andreas H Mahnken (Clinic of Diagnostic and Interventional Radiology, Marburg University, Marburg, Germany) enthuses to Interventional News about the potential of degradable starch microspheres (DSM) in transarterial chemoembolization, specifically drawing on his 20 years’ experience with DSM particles (PharmaCept). Indicated for chemoembolization of primary and secondary liver and lung tumours, EmboCept S DSM 50μm are the best-calibrated DSM available, ensuring good vasculature penetration and with a flexible application.
What is DSM-TACE and in which indications is it used?
Besides drug-eluting bead transarterial chemoembolization (DEB-TACE) and conventional transarterial chemoembolization (cTACE), there is a subset of TACE called DSM-TACE, where you use degradable starch microspheres roughly 50μm in size. These microspheres have a half-life of approximately 35 minutes, which means they are only around for a couple of hours at most. This allows you to re-access the same vessel for repeated TACE. The half-life is also short enough to prevent the proliferation of growth factors such as VEGF [vascular endothelial growth factor], which usually enter the bloodstream after a few hours of ischaemia. One of the major advantages of [microsphere] degradability is that you do not have this additional vascular growth. There are actually no limits to the indication within the liver, and you can also use the degradable starch microspheres in the lung for transpulmonary chemoembolization (DSM-TPCE) or in the bronchial arteries, so DSM-TACE has a really broad scope of indications. Our main focus in my department is for liver metastases and primary liver cancer, but we also use it for lung cancer.
What are the differences between DSM-TACE and cTACE/ DEB-TACE?
You have fewer limits. You can combine the procedure with any drug—not only, for example, doxorubicin or epirubicin, but you can really use it with any cytostatic that can be given intra-arterially, also in combinations, so it allows you to be much more flexible. That gives you a broader range of indications.
DSM-TACE also has excellent tolerability, so the patient experiences less pain. You can even perform a whole organ embolization with limited pain, which is pretty advantageous for the patient. It is much better-tolerated by the patient in terms of liver function and degradation, as well as in terms of pain. You can do it even in organs that would be critical for a cTACE or a DEB-TACE, so it gives you more flexibility—that is really the key for me.
If you have a patient with an organ that is already limited, for example a patient with cirrhosis, who then develops multinodular hepatocellular carcinoma (HCC), you may be concerned about having to treat multiple places in the liver. For example, with cTACE, there may be a limit because the function of the organ is not enough to allow treatment of all the tumours, but with DSM-TACE you can embolize all these tumours in a single session, and you will come out with enough remaining liver function to make sure that the patient can tolerate the procedure. In terms of efficacy, it is excellent.
Are there any limitations using DSM-TACE?
To the best of my knowledge there are no limitations. You can even combine it with cTACE; there is a very small prospective randomised trial that combines DSM-TACE with cTACE. That is another opportunity.
What are the future applications of DSM-TACE?
Future applications could include combining DSM-TACE with systemic or even regional immunotherapy—it can be combined with many new drugs. We are living in the time of immunotherapy, and DSM-TACE can be combined with several immune modulators. I feel that this is a really exciting possibility. We have preclinical data that shows us that there is T-cell homing in the tumour after DSM application, so that would be an ideal target for immune modulators, not only interleukins but modern drugs as well. I feel that is one of the greatest potentials DSM-TACE has for the next decade.
Case report
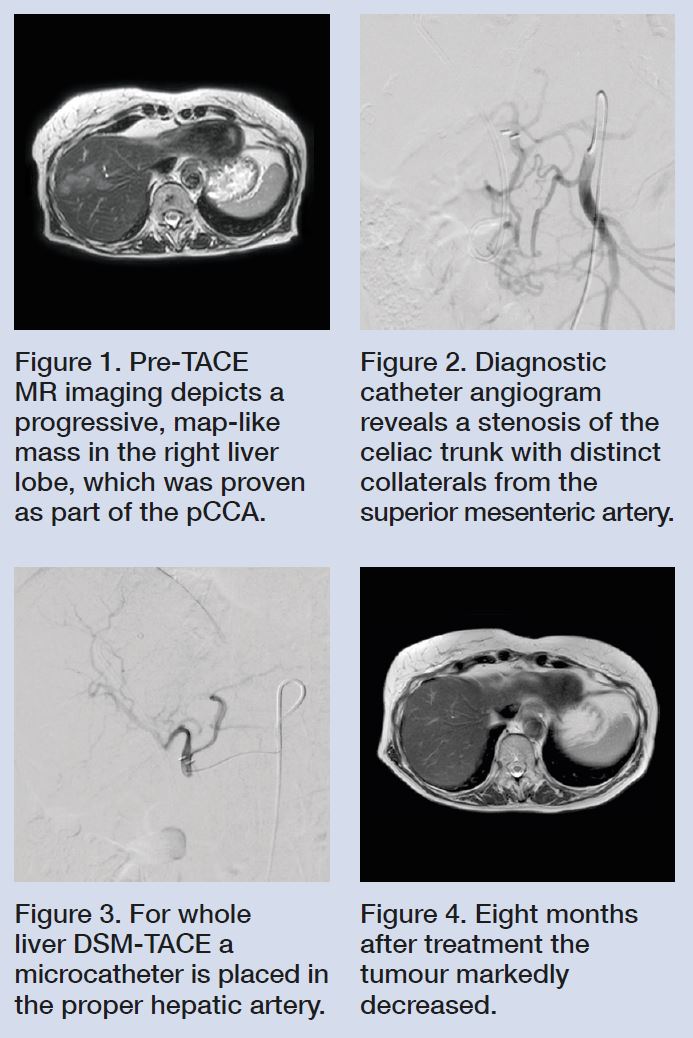 Cholangiocarcinoma is a highly lethal hepatic neoplasm with a median overall survival rate of about 16 months (Waseem 2017). Even after resection, the prognosis is poor, with reported three- and five-year survival rates after resection for perihilar cholangiocarcinoma (pCCA) of around 45% and 30% (Cillo 2019). In unresectable pCCA, common treatment options included chemotherapy with gemcitabine and cisplatinum, and transarterial chemoembolization (TACE).
Cholangiocarcinoma is a highly lethal hepatic neoplasm with a median overall survival rate of about 16 months (Waseem 2017). Even after resection, the prognosis is poor, with reported three- and five-year survival rates after resection for perihilar cholangiocarcinoma (pCCA) of around 45% and 30% (Cillo 2019). In unresectable pCCA, common treatment options included chemotherapy with gemcitabine and cisplatinum, and transarterial chemoembolization (TACE).
Patient history degradable
A 78-year-old female patient suffering from unresectable pCCA (Bismuth IV) was referred to our interventional radiology clinic for treatment after failure of first line chemotherapy. Magnetic resonance (MR) imaging revealed progression of the histologically-proven mass-forming pCCA, with multiple new histologies proving the presence of intrahepatic tumours and no extrahepatic tumour (Fig. 1).
On clinical examination, the patient was considered fit for interventional therapy. Laboratory testing showed mild anaemia and a moderately-elevated alkaline phosphatase. Her renal function was normal. She had previously undergone endoscopic biliary stenting, so her bilirubin was normal. We opted for a treatment with TACE and adjuvant capecitabine (Kelley 2020).
Interventional therapy degradable
Our TACE protocol for cholangiocarcinomas includes a so called DSM-TACE with 300mg degradable starch microspheres (EmboCept S DSM 50μm, PharmaCept, Berlin, Germany) combined with intra-arterial application of 75mg/m2 Cisplatinum (PlatiCept, PharmaCept, Berlin, Germany) and Gemcitabine (1,000mg/m2). In addition, a premedication for nausea, pain, and a single shot of intravenous (IV) antibiosis in case of biliodigestive- anastomosis or stenting.
Diagnostic catheter angiography at the beginning of the first treatment session showed a fixed high-grade stenosis of the celiac trunk with subsequent collateral flow from the superior mesenteric artery (Fig. 2). For whole liver treatment, a 2.7F microcatheter was placed in the proper hepatic artery and DSM-TACE with flow-controlled slow infusion of the chemotherapeutics was performed (Fig. 3). Unlike in conventional TACE (cTACE), drugs were infused over a 30-minute period in order to minimise gastrointestinal toxicity in terms of nausea and vomiting. During administration of chemotherapy, EmboCept S DSM 50μm was repeatedly applied under angiographic control via a three-way stopcock. In Germany, PlatiCept is the only available cisplatinum powder. Its major advantage is its good solubility. Therefore, relatively high doses of cisplatinum can be administered with a small injection volume. The latter is considered helpful for maintaining the embolic effect of TACE. With DSM such as EmboCept S DSM 50μm, repeat treatments are feasible, as the target lesion remains accessible. Moreover, the half-life time of about 35 minutes for EmboCept S DSM 50μm minimises systemic expression of proangiogenic growth factors such as vascular endothelial growth factor (VEGF) (Schicho 2016). It moreover reduces side effects such as ischaemic pain, thereby facilitating whole liver treatment.
At six week intervals, the patient underwent three successful DSM-TACE procedures. The procedures were well tolerated, with only a mild one-week fatigue as the only symptom of post-embolization syndrome. The eight-month follow-up MR imaging showed partial response according to RECIST 1.1, with only very little tumour left (Fig. 4). So far, no change on laboratory testing has been seen. Almost two years after the initial diagnosis, the patient feels well without limitations in her daily activity. She is now scheduled for a three-month treatment holiday followed restaging.
References:
1. Waseem D, Tushar P. Intrahepatic, perihilar and distal cholangiocarcinoma: Management and outcomes. Ann Hepatol. 2017 Jan–Feb 2017;16(1):133-139.
2. Kelley RK, Bridgewater J, Gores GJ et al. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. 2020 Feb;72(2):353–363
3. Cillo U, Fondevila C, Donadon M et al. Surgery for cholangiocarcinoma. Liver Int. 2019 May;39 Suppl 1(Suppl Suppl 1):143–155
4. Schicho A, Hellerbrand C, Krüger K et al. Impact of Different Embolic Agents for Transarterial Chemoembolization (TACE) Procedures on Systemic Vascular Endothelial Growth Factor (VEGF) Levels. J Clin Transl Hepatol. 2016 Dec 28;4(4):288–292











