In a comparison of the regenerative capacity of portal vein embolization (PVE) before major hepatectomies with two different embolic materials, N-butyl-cyanoacrylate (NBCA) with lipiodol was shown to be superior to polyvinyl-alcohol (PVA) particles plus coils. Speaking during the virtual European Conference of Interventional Oncology (ECIO; 4 November 2020, online), José Hugo Luz (Curry Cabral Hospital and Nova Medical School, Lisbon, Portugal) presented the results of the randomised, controlled BestFLR (Best future liver remnant) trial.
Liver regeneration strategies, such as PVE, are crucial in enabling patients to undergo major hepatectomies, Luz informed delegates. Contextualising the BestFLR trial, he went on to explain that PVE has gained acceptance as the standard of care for inducing liver growth in the last few decades. However, the optimal embolic material for PVE has not yet been established. “There are some suggestions in the literature that NBCA glue (Glubran, GEM) with Lipiodol (Guerbet) might generate more liver hypertrophy,” he said.
The BestFLR investigators therefore set out to determine which embolic material, NBCA-Lipiodol or PVA plus coils, produces the highest healthy liver growth during PVE. In order to be enrolled in the study, the future liver remnant (FLR)—the volume of healthy liver that will remain with the patient after surgery—had to be 25% or less of the total liver volume in healthy liver patients, 35% or less in colorectal patients, and 40 to 45% or less in patients with cirrhosis or cholestatic liver disease.
The 60 patients enrolled in the trial were randomly assigned to receive PVE with either NBCA-lipiodol or PVA plus coils (30 in each group). Baseline characteristics, such as age, sex, comorbidities, weight, height, and presence of cirrhosis, were equivalent amongst the two cohorts. The most common tumour types included in the study were colorectal metastases (45%) and cholangiocarcinoma (35%).
Detailing the embolization technique used by the triallists, Luz recounted: “We injected PVA particles of increasing size until [we reached] flow stasis, followed by proximal coils (6–12mm in diameter) to achieve complete venous occlusion”. For NBCA-Lipiodol, they injected a solution of different concentrations depending on the approach used: when injecting from the contralateral side, a 1:3 dilution was used, and when obtaining ipsilateral access to the liver, they used a 1:5 dilution through a microcatheter.
The primary outcome of the BestFLR trial was liver regeneration 14 and 28 days after PVE, assessed by computed tomography (CT) volumetry. The specific endpoints measured were FLR degree of hypertrophy, FLR absolute hypertrophy, and kinetic growth rate (KGR). Secondary outcomes included the occurrence of post-hepatectomy liver failure, intraoperative incidents, blood loss, transfusions, surgery duration and hospital stay, PVE contrast volume used, fluoroscopy and total procedure time, as well as the rate of minor and major complications after PVE. All patients gave voluntary informed consent to participate in the trial, which is registered in a World Health Organisation (WHO) registry network.
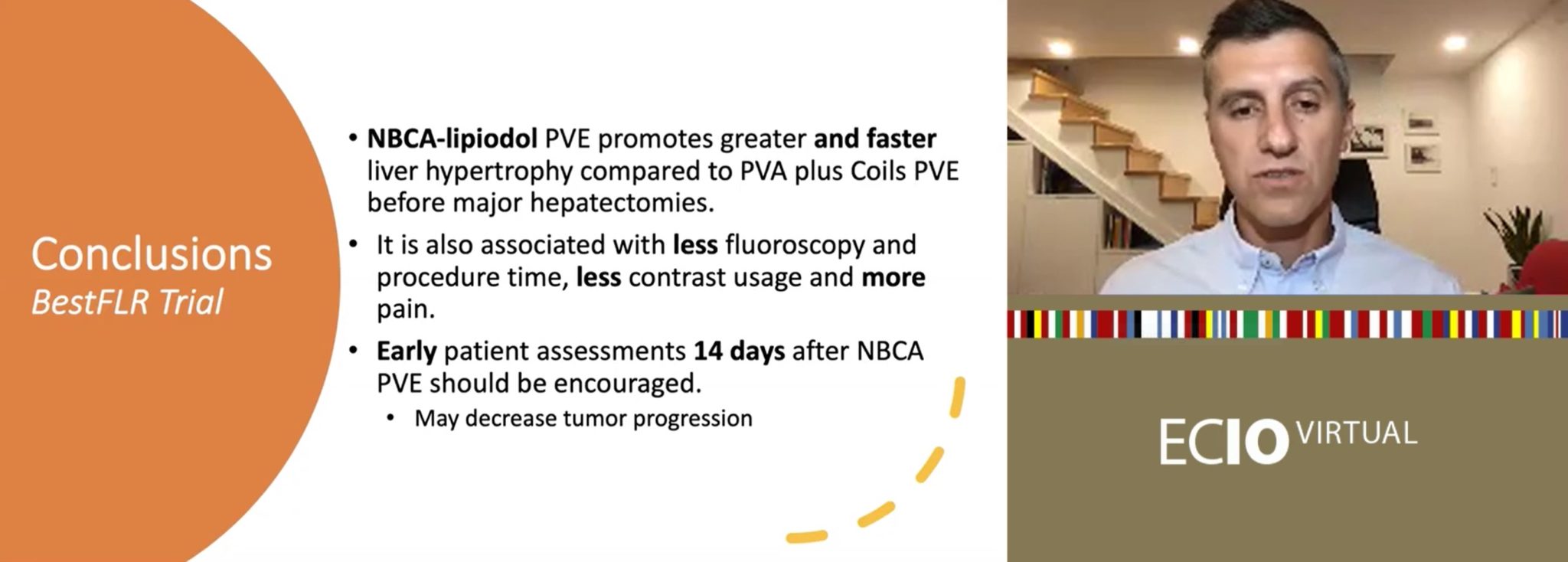
Triallists encourage earlier assessment of patients for hepatectomy in light of positive results at 14 days post-embolization with NBCA-lipiodol.
After 14 and 28 days, all volumetric parameters were superior for PVE with NBCA-Lipiodol compared to PVE with PVA plus coils, such as greater absolute liver hypertrophy of 46.4 versus 29.6, p<0.001 and 57.0 versus 36.7, p<0.001, respectively.
“An interesting finding that we had in this study is that patients were ready for surgery after just 14 days,” Luz said. In the NBCA-Lipiodol cohort, 87% of patients had a sufficient FLR ratio for liver surgery after 14 days. This was higher than amongst the PVA plus coils cohort, where 55% of patients were eligible for surgery after the same time period (p=0.008).
“These data encourage us to assess patients earlier,” Luz told the ECIO audience, “14 days after PVE if we are using NBCA-lipiodol, which we are now after the Trial. This shorter waiting period to enable patients to undergo curative hepatectomy might decrease tumour progression, which is the main cause of precluding liver surgery.”
Addressing patient outcomes, he shared that 24 patients (80%) in the NBCA-lipiodol group underwent a successful hepatectomy after PVE, compared to 23 (76%) in the PVA plus coils group (p=0.32). Three patients in the NBCA-lipiodol cohort (12%) and seven patients in the PVA plus coils cohort (27%) had clinically-relevant liver failure (p=0.27).
Fluoroscopy and total PVE time were also significantly lower for NBCA-lipiodol versus PVA plus coils (16 minutes vs. 25 minutes, p<0.001, and 60 minutes vs. 80 minutes, p=0.0023, respectively).
At the time of writing, the results of the BestFLR trial are under submission for publication.