This advertorial is sponsored by PharmaCept.
The Cardiovascular and Interventional Radiological Society of Europe (CIRSE) recently incorporated transarterial chemoembolization with degradable starch microspheres (DSM-TACE) into its guidelines for hepatic transarterial chemoembolization.¹ In addition to society backing, experts are also in favour of this treatment modality, underlining its role as a game-changing addition to the liver cancer care paradigm.
Speaking to Interventional News, Roberto Iezzi, associate professor of Radiology at the Catholic University of Rome (Rome, Italy) and Christiane Kuhl, head of the Department of Radiology at Aachen University (Aachen, Germany), share their clinical experiences with EmboCept® (PharmaCept®) and how the European guideline update solidifies its place in liver cancer treatment by TACE.

“EmboCept consists of a suspension of starch microspheres that are rapidly and completely degradable within 90–120 minutes² (in vitro half-life time 30–40 minutes) from the arterial infusion, allowing a temporary occlusion of the arterial microcirculation at the tumour level,” Iezzi explains, giving an overview of EmboCept’s mechanism of action. This, he says, improves the therapeutic effect by reducing the immediate wash-out of the cytostatic agent, and decreasing the risk of systemic toxicity and post-embolic syndrome.
EmboCept is also characterised by a cross-linked, partially hydrolysed starch matrix that allows the interventionalist to obtain a ready-to-use solution with different chemotherapeutic drugs being mixed with them. Iezzi recalls how this feature positively impacted his practice: “This option allowed us to open the door in the last few years for new indications, for liver metastases from lung, breast and gynaecological tumours, for example. You can now use EmboCept with any cytostatic that can be given intra-arterially, allowing [the interventionalist] to be much more flexible and broadening the range of indications.”

Kuhl notes that the current CIRSE recommendation reflects the current level of evidence, but is still conservative regarding the patient cohort included. She states: “Maintaining liver function is decisive for a patient’s treatment as well as survival. A patient needs a working liver, both to survive, but also because an adequate liver function is a prerequisite for most systemic treatment protocols.” In patients with metastatic solid tumours, metastatic overgrowth of the liver can thus become the driver of a patient’s prognosis. Kuhl highlights that interventionalists now have the tools to help the liver fight against this metastatic overgrowth in addition to systemic treatment. “With systemic treatment, often in the liver, the dose is simply not high enough to stop the cancer,” she says, stressing that in these patients, palliation of liver function through local treatment is key, and is the major goal of liver-directed treatment. “This is similar to the local treatment of osseous metastasis to a vertebral body, where the intention is to palliate the static function of the bone,” Kuhl adds. “Similarly, in patients with secondary liver cancer, the aim is to palliate the liver. We are able to do that with EmboCept-driven transarterial chemotherapy”.
In the field of embolic agents, both interventionalists are in agreement that EmboCept holds its ground, providing a solid treatment option and in some cases offering advantages over alternatives. “I think the most obvious advantage of EmboCept compared with other embolic agents is the fact that it only causes a temporary occlusion, maintaining the route to the tumour,” Kuhl opines. She believes this is crucial in the treatment of liver cancer other than hepatocellular carcinoma (HCC), where the main method of action may not be devascularisation, but in bringing the chemotherapeutic agents into the tumour. Kuhl elaborates: “Everywhere we want to treat chemo-sensitive cancer in the liver, these agents must be applied not only once, but repetitively—just as is usual practice with their systemic use. In such patients, temporary occlusion of vessels by degradable starch microspheres allows one to keep the route to the tumour patent for several courses of transarterial chemotherapy.” Such TACE should only temporarily occlude the vessels to avoid passage of the chemotherapeutic or cytotoxic agent through the tumour, but ideally keep it inside the tumour.” This is what EmboCept achieves in a perfect way,” Kuhl remarks.
According to Iezzi, DSM-TACE can offer benefits where other TACE options, namely drug-eluting bead TACE (DEB-TACE) and conventional TACE (cTACE), are not indicated. “DSM-TACE is safe, without side effects or worsening of liver function. It is also safe in advanced HCC patients, with the worst clinical conditions (Child-Pugh>B7), vascular invasion (portal vein thrombosis), high bilirubin level or ascites—patients in whom DEB or cTACE could be contraindicated,” he relays.
Overall, however, it is Iezzi’s opinion that DSM-TACE is not necessarily in competition with DEB or cTACE, but provides another treatment option that interventionalists can offer to their patients, thereby widening clinical indications and improving disease prognosis overall. Iezzi adds that DSM-TACE “could also be used as a bridge to other locoregional or surgical options as it is effective and repeatable (so called bridging and downstaging) being able to maintain vessel patency”.
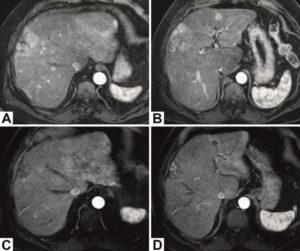
CIRSE’s approval of DSM-TACE has solidified its place as “an important pillar of tumour treatment,” says Kuhl. In recent years, a number of papers have been published that highlight the role of DSM-TACE in HCC, and all of these have contributed to the acceptance of DSM-TACE in the CIRSE guidelines. For example, Iezzi was part of a team that recently published a multicentre European study confirming that DSM-TACE is well tolerated with no major clinical adverse events and only limited major laboratory events.³ He tells Interventional News that preserved liver function was observed after repetitive DSM-TACE treatments in this study. “Our results confirm that repetitive DSM-TACE is a safe, well tolerated and effective treatment option for HCC patients with high tumour burden, ineligible for, or failing other palliative therapies,” said Iezzi, relaying the key take-away message from the paper.
“Guidelines are what drive clinical patient care,” Kuhl states, underlining the significance of the CIRSE update. DSM-TACE is now an accredited treatment option and this, according to Iezzi, “widens indications for locoregional treatments and gives new opportunities to patients”. In addition, he believes that the guideline update might convince all interventional radiologists, hepatologists and oncologists who were doubtful of the important role of DSM-TACE in the treatment of liver cancer.
References
1. Lucatelli P, Burrel M, Guiu B, et al. CIRSE standards of practice on hepatic transarterial chemoembolisation. Cardiovasc Intervent Radiol. 2021; 44 (12): 1851–1867. DOI: 10.1007/s00270-021-02968-1.
2. Wiggermann, P, Heibl M, Niessen C, et al. Degradable starch microspheres transarterial chemoembolisation (DSM-TACE) of HCC: dynamic contrast-enhanced ultrasonography (DCE-US) based evaluation of therapeutic efficacy using a novel perfusion software. Clin. Hemorheol. Microcirc. 2012; 52 (2–4): 123–129. DOI: 10.3233/CH-2012-1590.
3. Ludwig, JM, Iezzi R, Theysohn JM, et al. European multicenter study on degradable starch microsphere TACE. The digestible way to conquer HCC in patients with High tumor burden. Cancers. 2021; 13 (20).













